Carnahan-Starling equation of state: Difference between revisions
mNo edit summary |
(Added the errors given by Clisby and McCoy to the table of virial coefficients.) |
||
| (46 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
The | [[Image:CS_EoS_plot.png|thumb|350px|right]] | ||
The '''Carnahan-Starling equation of state''' is an approximate (but quite good) [[Equations of state |equation of state]] for the fluid phase of the [[hard sphere model]] in three dimensions. It is given by (Ref <ref name="CH"> [http://dx.doi.org/10.1063/1.1672048 N. F. Carnahan and K. E. Starling,"Equation of State for Nonattracting Rigid Spheres" Journal of Chemical Physics '''51''' pp. 635-636 (1969)] </ref> Eqn. 10). | |||
: <math> | : <math> | ||
| Line 6: | Line 7: | ||
where: | where: | ||
*<math> Z </math> is the [[compressibility factor]] | |||
*<math> p </math> is the [[pressure]] | |||
*<math> V </math> is the volume | |||
*<math> N </math> is the number of particles | |||
*<math> k_B </math> is the [[Boltzmann constant]] | |||
*<math> T </math> is the absolute [[temperature]] | |||
*<math> \eta </math> is the [[packing fraction]]: | |||
:<math> \eta = \frac{ \pi }{6} \frac{ N \sigma^3 }{V} </math> | |||
*<math> \sigma </math> is the [[hard sphere model | hard sphere]] diameter. | |||
The Carnahan-Starling equation of state is not applicable for packing fractions greater than 0.55 <ref>[https://arxiv.org/abs/cond-mat/0605392 Hongqin Liu "A very accurate hard sphere equation of state over the entire stable and metstable region", arXiv:cond-mat/0605392 (2006)]</ref>. | |||
==Virial expansion== | |||
It is interesting to compare the [[Virial equation of state | virial coefficients]] of the Carnahan-Starling equation of state (Eq. 7 of <ref name="CH"></ref>) with the [[Hard sphere: virial coefficients | hard sphere virial coefficients]] in three dimensions (exact up to <math>B_4</math>, and those of Clisby and McCoy <ref> [http://dx.doi.org/10.1007/s10955-005-8080-0 Nathan Clisby and Barry M. McCoy "Ninth and Tenth Order Virial Coefficients for Hard Spheres in D Dimensions", Journal of Statistical Physics '''122''' pp. 15-57 (2006)] </ref>): | |||
{| style="width:40%; height:100px" border="1" | |||
|- | |||
| <math>n</math> ||Clisby and McCoy ||<math>B_n=n^2+n-2</math> | |||
|- | |||
| 2 || 4 || 4 | |||
|- | |||
| 3 || 10 || 10 | |||
|- | |||
| 4 || 18.3647684 || 18 | |||
|- | |||
| 5 || 28.22451(26) || 28 | |||
|- | |||
| 6 || 39.81515(93) || 40 | |||
|- | |||
| 7 || 53.3444(37) || 54 | |||
|- | |||
| 8 || 68.538(18) || 70 | |||
|- | |||
| 9 || 85.813(85) || 88 | |||
|- | |||
| 10 || 105.78(39) || 108 | |||
|} | |||
==Thermodynamic expressions== | |||
From the Carnahan-Starling equation for the fluid phase | |||
the following thermodynamic expressions can be derived | |||
(Ref <ref>[http://dx.doi.org/10.1063/1.469998 Lloyd L. Lee "An accurate integral equation theory for hard spheres: Role of the zero-separation theorems in the closure relation", Journal of Chemical Physics '''103''' pp. 9388-9396 (1995)]</ref> Eqs. 2.6, 2.7 and 2.8) | |||
[[Pressure]] (compressibility): | |||
:<math>\frac{p^{CS}V}{N k_B T } = \frac{1+ \eta + \eta^2 - \eta^3}{(1-\eta)^3}</math> | |||
Configurational [[chemical potential]]: | |||
:<math>\frac{ \overline{\mu }^{CS}}{k_B T} = \frac{8\eta -9 \eta^2 + 3\eta^3}{(1-\eta)^3}</math> | |||
Isothermal [[compressibility]]: | |||
:<math>\chi_T -1 = \frac{1}{k_BT} \left.\frac{\partial P^{CS}}{\partial \rho}\right\vert_{T} -1 = \frac{8\eta -2 \eta^2 }{(1-\eta)^4}</math> | |||
where <math>\eta</math> is the [[packing fraction]]. | |||
Configurational [[Helmholtz energy function]]: | |||
:<math> \frac{ A_{ex}^{CS}}{N k_B T} = \frac{4 \eta - 3 \eta^2 }{(1-\eta)^2}</math> | |||
==The 'Percus-Yevick' derivation== | |||
It is interesting to note (Ref <ref> [http://dx.doi.org/10.1063/1.1675048 G. A. Mansoori, N. F. Carnahan, K. E. Starling, and T. W. Leland, Jr. "Equilibrium Thermodynamic Properties of the Mixture of Hard Spheres", Journal of Chemical Physics '''54''' pp. 1523-1525 (1971)] </ref> Eq. 6) that one can arrive at the Carnahan-Starling equation of state by adding two thirds of the [[exact solution of the Percus Yevick integral equation for hard spheres]] via the compressibility route, to one third via the pressure route, i.e. | |||
:<math>Z = \frac{ p V}{N k_B T} = \frac{2}{3} \left[ \frac{(1+\eta+\eta^2)}{(1-\eta)^3} \right] + \frac{1}{3} \left[ \frac{(1+2\eta+3\eta^2)}{(1-\eta)^2} \right] = \frac{ 1 + \eta + \eta^2 - \eta^3 }{(1-\eta)^3 }</math> | |||
The reason for this seems to be a slight mystery (see discussion in Ref. <ref>[http://dx.doi.org/10.1021/j100356a008 Yuhua Song, E. A. Mason, and Richard M. Stratt "Why does the Carnahan-Starling equation work so well?", Journal of Physical Chemistry '''93''' pp. 6916-6919 (1989)]</ref> ). | |||
== Kolafa correction == | |||
Jiri Kolafa produced a slight correction to the C-S EOS which results in improved accuracy <ref>[http://dx.doi.org/10.1063/1.4870524 Miguel Robles, Mariano López de Haro and Andrés Santos "Note: Equation of state and the freezing point in the hard-sphere model", Journal of Chemical Physics '''140''' 136101 (2014)]</ref>: | |||
:<math> \eta = \frac{ \ | : <math> | ||
Z = \frac{ 1 + \eta + \eta^2 - \frac{2}{3}(1+\eta) \eta^3 }{(1-\eta)^3 }. | |||
</math> | |||
== Liu correction == | |||
Hongqin Liu proposed a correction to the C-S EOS which improved accuracy by almost two order of magnitude <ref>[https://arxiv.org/abs/2010.14357 Hongqin Liu "Carnahan Starling type equations of state for stable hard disk and hard sphere fluids", arXiv:2010.14357]</ref>: | |||
: <math> | |||
Z = \frac{ 1 + \eta + \eta^2 - \frac{8}{13}\eta^3 - \eta^4 + \frac{1}{2}\eta^5 }{(1-\eta)^3 }. | |||
</math> | |||
== See also == | |||
*[[Equations of state for hard spheres]] | |||
*[[Kolafa-Labík-Malijevský equation of state]] | |||
== References == | |||
<references/> | |||
[[Category: Equations of state]] | |||
[[category: hard sphere]] | |||
Latest revision as of 14:25, 21 March 2023
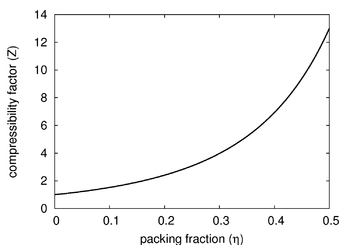
The Carnahan-Starling equation of state is an approximate (but quite good) equation of state for the fluid phase of the hard sphere model in three dimensions. It is given by (Ref [1] Eqn. 10).
where:
- is the compressibility factor
- is the pressure
- is the volume
- is the number of particles
- is the Boltzmann constant
- is the absolute temperature
- is the packing fraction:
- is the hard sphere diameter.
The Carnahan-Starling equation of state is not applicable for packing fractions greater than 0.55 [2].
Virial expansion[edit]
It is interesting to compare the virial coefficients of the Carnahan-Starling equation of state (Eq. 7 of [1]) with the hard sphere virial coefficients in three dimensions (exact up to , and those of Clisby and McCoy [3]):
| Clisby and McCoy | ||
| 2 | 4 | 4 |
| 3 | 10 | 10 |
| 4 | 18.3647684 | 18 |
| 5 | 28.22451(26) | 28 |
| 6 | 39.81515(93) | 40 |
| 7 | 53.3444(37) | 54 |
| 8 | 68.538(18) | 70 |
| 9 | 85.813(85) | 88 |
| 10 | 105.78(39) | 108 |
Thermodynamic expressions[edit]
From the Carnahan-Starling equation for the fluid phase the following thermodynamic expressions can be derived (Ref [4] Eqs. 2.6, 2.7 and 2.8)
Pressure (compressibility):
Configurational chemical potential:
Isothermal compressibility:
where is the packing fraction.
Configurational Helmholtz energy function:
The 'Percus-Yevick' derivation[edit]
It is interesting to note (Ref [5] Eq. 6) that one can arrive at the Carnahan-Starling equation of state by adding two thirds of the exact solution of the Percus Yevick integral equation for hard spheres via the compressibility route, to one third via the pressure route, i.e.
The reason for this seems to be a slight mystery (see discussion in Ref. [6] ).
Kolafa correction[edit]
Jiri Kolafa produced a slight correction to the C-S EOS which results in improved accuracy [7]:
Liu correction[edit]
Hongqin Liu proposed a correction to the C-S EOS which improved accuracy by almost two order of magnitude [8]:
See also[edit]
References[edit]
- ↑ 1.0 1.1 N. F. Carnahan and K. E. Starling,"Equation of State for Nonattracting Rigid Spheres" Journal of Chemical Physics 51 pp. 635-636 (1969)
- ↑ Hongqin Liu "A very accurate hard sphere equation of state over the entire stable and metstable region", arXiv:cond-mat/0605392 (2006)
- ↑ Nathan Clisby and Barry M. McCoy "Ninth and Tenth Order Virial Coefficients for Hard Spheres in D Dimensions", Journal of Statistical Physics 122 pp. 15-57 (2006)
- ↑ Lloyd L. Lee "An accurate integral equation theory for hard spheres: Role of the zero-separation theorems in the closure relation", Journal of Chemical Physics 103 pp. 9388-9396 (1995)
- ↑ G. A. Mansoori, N. F. Carnahan, K. E. Starling, and T. W. Leland, Jr. "Equilibrium Thermodynamic Properties of the Mixture of Hard Spheres", Journal of Chemical Physics 54 pp. 1523-1525 (1971)
- ↑ Yuhua Song, E. A. Mason, and Richard M. Stratt "Why does the Carnahan-Starling equation work so well?", Journal of Physical Chemistry 93 pp. 6916-6919 (1989)
- ↑ Miguel Robles, Mariano López de Haro and Andrés Santos "Note: Equation of state and the freezing point in the hard-sphere model", Journal of Chemical Physics 140 136101 (2014)
- ↑ Hongqin Liu "Carnahan Starling type equations of state for stable hard disk and hard sphere fluids", arXiv:2010.14357

















![{\displaystyle Z={\frac {pV}{Nk_{B}T}}={\frac {2}{3}}\left[{\frac {(1+\eta +\eta ^{2})}{(1-\eta )^{3}}}\right]+{\frac {1}{3}}\left[{\frac {(1+2\eta +3\eta ^{2})}{(1-\eta )^{2}}}\right]={\frac {1+\eta +\eta ^{2}-\eta ^{3}}{(1-\eta )^{3}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/be142b5b421225e5516478eb7d6d109e06d8dd61)

