SPC/E model of water: Difference between revisions
Oliveirajs (talk | contribs) mNo edit summary |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Stub-water}} | {{Stub-water}} | ||
The '''SPC/E''' (extended simple point charge model) | The '''SPC/E''' (extended simple point charge model) <ref>[http://dx.doi.org/10.1021/j100308a038 H. J. C. Berendsen, J. R. Grigera, and T. P. Straatsma "The missing term in effective pair potentials", Journal of Physical Chemistry '''91''' pp. 6269 - 6271 (1987)]</ref> | ||
<ref>[http://dx.doi.org/10.1063/1.2841127 Swaroop Chatterjee, Pablo G. Debenedetti, Frank H. Stillinger, and Ruth M. Lynden-Bell "A computational investigation of thermodynamics, structure, dynamics and solvation behavior in modified water models", Journal of Chemical Physics '''128''' 124511 (2008)]</ref> is a slight reparameterisation of the [[SPC]] model of [[water]], with a modified value for <math>q_{\mathrm{O}}</math>. | |||
The molecule is modelled as | The molecule is modelled as | ||
a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range [[Lennard-Jones model | Lennard-Jones]] sites, situated on the oxygen atoms. The parameters are as follows: | a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range [[Lennard-Jones model | Lennard-Jones]] sites, situated on the oxygen atoms. The parameters are as follows: | ||
| Line 9: | Line 9: | ||
| parameter || value | | parameter || value | ||
|- | |- | ||
| | | <math>\sigma</math> || <math> 3.166 </math> Å | ||
|- | |- | ||
| | | <math>\epsilon</math> || <math>0.650</math> kJ mol<sup>-1</sup> | ||
|- | |- | ||
| | | <math>r_\mathrm{OH}</math> || <math>1.000</math> Å | ||
|- | |- | ||
| | | <math>\angle_\mathrm{HOH}</math> || <math>109.47^{\circ}</math> | ||
|- | |- | ||
| | | <math>q_{\mathrm{O}}</math> || <math>-0.8476 e</math> | ||
|- | |- | ||
| | | <math>q_{\mathrm{H}}</math> || <math>|q_{\mathrm{O}}|/2</math> (charge neutrality) | ||
|} | |} | ||
The SPC/E model has a [[dipole moment]] of 2.351 D. (Ref. 1 Table I). | The SPC/E model has a [[dipole moment]] of 2.351 D. (Ref. 1 Table I). | ||
==Surface tension== | ==Surface tension== | ||
The [[surface tension]] has been studied for the SPC/E model by Vega and Miguel | The [[surface tension]] has been studied for the SPC/E model by Vega and Miguel | ||
<ref>[http://dx.doi.org/10.1063/1.2715577 C. Vega and E. de Miguel "Surface tension of the most popular models of water by using the test-area simulation method", Journal of Chemical Physics '''126''' 154707 (2007)]</ref> | |||
==Phase diagram== | ==Phase diagram== | ||
[[Image:SPC_E_phase_diagram.png|right|400px]] | [[Image:SPC_E_phase_diagram.png|right|400px]] | ||
===Plastic crystal phases=== | ===Plastic crystal phases=== | ||
Recent simulations have demonstrated the existence of [[Plastic crystals | plastic crystal]] phases for the SPC/E model. | Recent simulations have demonstrated the existence of [[Plastic crystals | plastic crystal]] phases for the SPC/E model. | ||
<ref>[http://dx.doi.org/10.1063/1.3156856 J. L. Aragones and C. Vega "Plastic crystal phases of simple water models", Journal of Chemical Physics '''130''' 244504 (2009)]</ref> | |||
<ref>[https://doi.org/10.1021/acs.jpcb.1c05053 I. Skarmoutsos, A. Henao, J. Samios and E. Guardia "On the Different Faces of the Supercritical Phase of Water at a Near-Critical Temperature: Pressure-Induced Structural Transitions Ranging from a Gaslike Fluid to a Plastic Crystal Polymorph", Journal of Physical Chemistry B '''125''' 10260 (2022)]</ref> | |||
==Shear viscosity== | ==Shear viscosity== | ||
The [[shear viscosity]] for the SPC/E model is 0.729 mPa.s at 298 K and 1 bar | The [[shear viscosity]] for the SPC/E model is 0.729 mPa.s at 298 K and 1 bar <ref>[http://dx.doi.org/10.1063/1.3330544 Miguel Angel González and José L. F. Abascal "The shear viscosity of rigid water models", Journal of Chemical Physics '''132''' 096101 (2010)]</ref> (experimental value 0.896 mPa.s <ref>[http://dx.doi.org/10.1021/je049918m Kenneth R. Harris and Lawrence A. Woolf "Temperature and Volume Dependence of the Viscosity of Water and Heavy Water at Low Temperatures", Journal of Chemical & Engineering Data '''49''' pp. 1064-1069 (2004)]</ref>). | ||
==Thermal conductivity== | ==Thermal conductivity== | ||
[[Thermal conductivity]] | [[Thermal conductivity]] <ref>[http://dx.doi.org/10.1063/1.4739855 Frank Römer, Anders Lervik, and Fernando Bresme "Nonequilibrium molecular dynamics simulations of the thermal conductivity of water: A systematic investigation of the SPC/E and TIP4P/2005 models", Journal of Chemical Physics '''137''' 074503 (2012)]</ref>. | ||
==References== | ==References== | ||
<references/> | |||
;Related reading | ;Related reading | ||
*[http://dx.doi.org/10.1063/1.3548869 Péter T. Kiss and András Baranyai "Sources of the deficiencies in the popular SPC/E and TIP3P models of water", Journal of Chemical Physics '''134''' 054106 (2011)] | *[http://dx.doi.org/10.1063/1.3548869 Péter T. Kiss and András Baranyai "Sources of the deficiencies in the popular SPC/E and TIP3P models of water", Journal of Chemical Physics '''134''' 054106 (2011)] | ||
[[category: water]] | [[category: water]] | ||
[[category: models]] | [[category: models]] | ||
Latest revision as of 16:10, 23 November 2023
The SPC/E (extended simple point charge model) [1] [2] is a slight reparameterisation of the SPC model of water, with a modified value for . The molecule is modelled as a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range Lennard-Jones sites, situated on the oxygen atoms. The parameters are as follows:
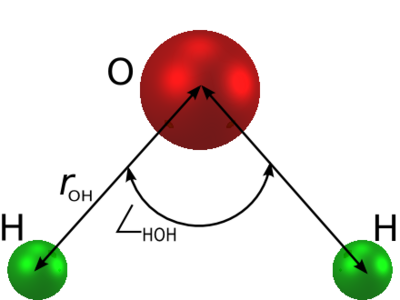
| parameter | value |
| Å | |
| kJ mol-1 | |
| Å | |
| (charge neutrality) |
The SPC/E model has a dipole moment of 2.351 D. (Ref. 1 Table I).
Surface tension[edit]
The surface tension has been studied for the SPC/E model by Vega and Miguel [3]
Phase diagram[edit]

Plastic crystal phases[edit]
Recent simulations have demonstrated the existence of plastic crystal phases for the SPC/E model. [4] [5]
Shear viscosity[edit]
The shear viscosity for the SPC/E model is 0.729 mPa.s at 298 K and 1 bar [6] (experimental value 0.896 mPa.s [7]).
Thermal conductivity[edit]
References[edit]
- ↑ H. J. C. Berendsen, J. R. Grigera, and T. P. Straatsma "The missing term in effective pair potentials", Journal of Physical Chemistry 91 pp. 6269 - 6271 (1987)
- ↑ Swaroop Chatterjee, Pablo G. Debenedetti, Frank H. Stillinger, and Ruth M. Lynden-Bell "A computational investigation of thermodynamics, structure, dynamics and solvation behavior in modified water models", Journal of Chemical Physics 128 124511 (2008)
- ↑ C. Vega and E. de Miguel "Surface tension of the most popular models of water by using the test-area simulation method", Journal of Chemical Physics 126 154707 (2007)
- ↑ J. L. Aragones and C. Vega "Plastic crystal phases of simple water models", Journal of Chemical Physics 130 244504 (2009)
- ↑ I. Skarmoutsos, A. Henao, J. Samios and E. Guardia "On the Different Faces of the Supercritical Phase of Water at a Near-Critical Temperature: Pressure-Induced Structural Transitions Ranging from a Gaslike Fluid to a Plastic Crystal Polymorph", Journal of Physical Chemistry B 125 10260 (2022)
- ↑ Miguel Angel González and José L. F. Abascal "The shear viscosity of rigid water models", Journal of Chemical Physics 132 096101 (2010)
- ↑ Kenneth R. Harris and Lawrence A. Woolf "Temperature and Volume Dependence of the Viscosity of Water and Heavy Water at Low Temperatures", Journal of Chemical & Engineering Data 49 pp. 1064-1069 (2004)
- ↑ Frank Römer, Anders Lervik, and Fernando Bresme "Nonequilibrium molecular dynamics simulations of the thermal conductivity of water: A systematic investigation of the SPC/E and TIP4P/2005 models", Journal of Chemical Physics 137 074503 (2012)
- Related reading











